Klaus Jaffe and Roberto Cipriani (2007)
Culture Outsmarts Nature in the Evolution of Cooperation
Journal of Artificial Societies and Social Simulation
vol. 10, no. 1
<https://www.jasss.org/10/1/7.html>
For information about citing this article, click here
Received: 09-Apr-2006 Accepted: 16-Nov-2006 Published: 31-Jan-2007
 Abstract
Abstract| Table 1: Average proportion of collaborators (co), competing against non collaborators (nco) and free riders (fr), resulting from scenarios in which cooperation has no cost to co, and genetic and non genetic information is transmitted from parents to offspring and from peers to peers with different mechanisms: G, vertical transmission of information using genes; H, horizontal transmission of non genetic information; C, horizontal and vertical transmission of non genetic information; and Control runs, in which transmission of information never occurs. All standard deviations are smaller than 1.5%. Selection settings: p0n = pnco = 0.5 and p2n = p1n = 0.1. Shuffling: agent's locations were randomly swapped; Heredity: the proportion of newborn is the same as that of the surviving parents in previous time step. Without heredity, rco, rnco, and rfr = 0.5. | ||||
| Exp. | Scenarios | Shuffling | Heredity | Proportion of co |
| 1 | G | Y | Y | 1.00 |
| co vs nco | H | Y | N | 0.99 |
| C | Y | Y | 1.00 | |
| Control | Y | N | 0.88 | |
| 2 | G | N | Y | 1.00 |
| co vs nco | H | N | N | 0.98 |
| C | N | Y | 1.00 | |
| Control | N | N | 0.89 | |
| 3 | G | Y | Y | 0 or 1 |
| co vs fr | H | Y | N | 0.99 |
| C | Y | Y | 1.00 | |
| Control | Y | N | 0.50 | |
| 4 | G | N | Y | 1.00 |
| co vs fr | H | N | N | 0.94 |
| C | N | Y | 1.00 | |
| Control | N | N | 0.52 | |
|
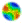
| Figure 1. Average proportion of collaborators (co), competing against non collaborators (nco) and free riders (fr), resulting from scenarios in which cooperation had a cost on co, and information was transmitted using different mechanisms: G, vertical transmission of information using genes, large white circles; H, horizontal transmission of non genetic information, white squares; C, horizontal and vertical transmission of non genetic information, small black circles; and Control, without transmission of information, white triangles. A. co vs. nco with random shuffling of agent's locations; B. co vs. nco without random shuffling; C. co vs. fr with random shuffling. The proportion of co under G oscillated between 0 and 1 (not shown); D. co vs. fr without random shuffling. All standard deviations are smaller than 1.5%. Selection settings: p0n = pnco = 0.5 and p2n = p1n = 0.1. Costs on cooperators: mco = 0, 0.1, 0.2, ..., 0.9, 1 and mnco = mfr = 0. |
|
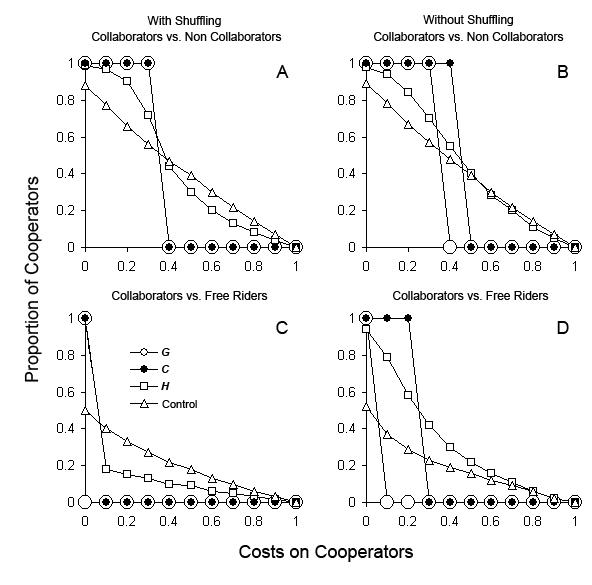
| Figure 2. Range of cooperation costs in which the dominance in populations switched from collaborators to competing strategies in G and C, under different values of selection against grouping agents. Experiments simulate collaborators (co) competing against non collaborators (nco) and free riders (fr) under two different scenarios: G, vertical transmission of information using genes, gray bars; C, horizontal and vertical transmission of non genetic information, black bars. A. co vs. nco with random shuffling of agent's locations; B. co vs. nco without random shuffling; C. co vs. fr with random shuffling; D. co vs. fr without random shuffling. Selection settings: p0n = pnco = 0.2, 0.3, &hellip, 0.9 and p2n = p1n = 0.1. Costs on cooperators: mco = 0, 0.1, 0.2, …, 0.9, 1 and mnco = mfr = 0. |
|
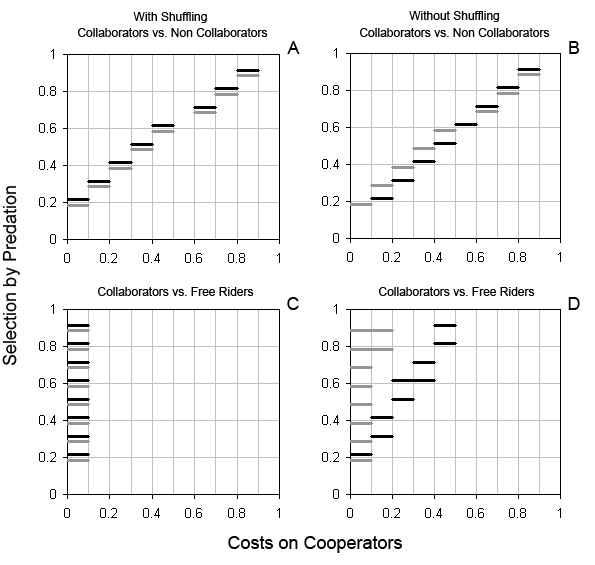
| Figure 3. Average proportion of collaborators (co), competing against non collaborators (nco) and free riders (fr), resulting from scenarios in which cooperation had a cost on co, and information was transmitted using different mechanisms: H, horizontal transmission of non genetic information, squares; Control, without transmission of information, triangles. A. co vs. nco with random shuffling of agent's locations; B. co vs. nco without random shuffling; C. co vs. fr with random shuffling; D. co vs. fr without random shuffling. All standard deviations are smaller than 1.5%. Fitness advantages for cooperation: Low selection: p0n = pnco = 0.2 and p2n = p1n = 0.1, white symbols; High selection p0n = pnco = 0.9 and p2n = p1n = 0.1, black symbols. Costs on cooperators: mco = 0, 0.1, 0.2, ..., 0.9, 1 and mnco = mfr = 0. |
|
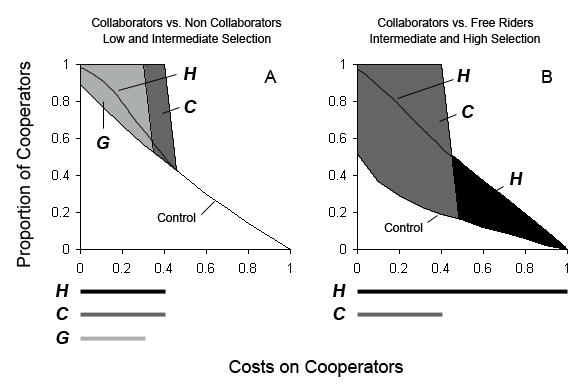
| Figure 4. Schematic representation of scenarios linked to cultural evolution, C and H, allowing collaborators (co) to dominate populations at cooperation costs higher than those allowed by the scenario representing biological evolution, G. Darkened areas represent the cost differential attributed to cultural evolution that benefits the dominance of co. The lower limits of darkened areas are the co values resulting from control runs. Bars at the bottom of each plot show the extent of costs along which different scenarios allow co to become the dominant strategy. G, vertical transmission of information using genes, gray; C, horizontal and vertical transmission of non genetic information, dark gray; H, horizontal transmission of non genetic information, black. A. co competing against non collaborators (nco) without random shuffling of agent's locations, under an intermediate fitness advantage for grouping or intermediate selection (e.g., p0n = pnco = 0.5) on agents that do not form groups; B. co competing against free riders (fr) without random shuffling, under a strong selection (e.g., p0n = pnco = 0.9) on agents that do not form groups. Costs on cooperators: mco = 0, 0.1, 0.2, …, 0.9, 1 and mnco = mfr = 0. |
ANDERLINI L and Ianni A (1996) Path dependence and learning from neighbours. Games and Economic Behavior, vol. 13, pp. 141-177.
AXELROD R (1997) The Dissemination of culture. A model with local convergence and global polarization. Journal of Conflict Resolution, vol. 41 no. 2, pp. 203-226.
AXELROD R (1989) The Evolution of Cooperation. Penguin, Harmondsworth.
AXELROD R and Hamilton W D (1981) The evolution of cooperation. Science, vol. 211, pp. 1390-96.
BEST M (1999) How Culture Can Guide Evolution: An inquiry into gene/meme enhancement and opposition. Adaptative Behaviour, vol. 7, pp. 289-306.
BULL L, Holland O. and Blackmore S (2000) On Meme-Gene Coevolution. Artificial Life. vol. 6, pp. 227-35.
BOYD R and Richerson P (1985) Culture and the evolutionary process. Chicago: University of Chicago Press.
BOYD R. and Richerson P (1996) Why culture is common but cultural evolution is rare. Proceedings of the British Academy 88: 73-93.
CAVALLI-SFORZA L.L. and Feldman M.W (1981) Cultural transmission and evolution: a quantitative approach. Monogr Popul Biol. 16:1-388.
CAVALLI-SFORZA L.L. and Feldman M.W (2003) The application of molecular genetic approaches to the study of human evolution, Nature Genetics 33: 266-27
CIPRIANI R and Jaffe K (2005) On the dynamics of grouping. p. 56-60. In: G. Tonella, ed. Proceedings of the Fifth IASTED International Conference on Modelling, Simulation and Optimization, Acta Press.
DAWKINS R (1976) The Selfish Gene. Oxford Univ. Press, Oxford
DEWAAL F.B.M (2001) The ape and the sushi master: Cultural reflections of a primatologist. Basic Books. New York.
EHRLICH P.R and Levin S.A (2005) The Evolution of Norms. PLoS Biology, 3 (6) e194
FEHR E and Gachter S (2002) Altruistic punishment in humans. Nature 415, 137-140.
FEHR E and Fischbauer U (2003) The nature of human altruism. Nature 425, 785-791.
GADAGKAR R (2001) The social biology of Ropalidia marginata: Towards understanding the evolution of eusociality. Harvard University Press.
HAMILTON W.D (1964) The genetic evolution of social behaviour. Papers I and II. Journal of Theoretical Biology 7: 1-16, 17-52.
HAMILTON W.D (1971) Geometry for the selfish herd. Journal of Theoretical Biology 31: 295-311.
HAMMERSTEIN P (ed.) (2004) Genetic and cultural evolution of cooperation. Dahlem Workshop Reports, MIT Press. 450 p.
HENRICH J and Boyd R (1998) The Evolution of Conformist Transmission and the Emergence of Between-Group Differences. Evolution and Human Behavior 19: 215-242.
IFTIA M, Killingback T, Doebeli M (2004) Effects of neighborhood size and connectivity on the spatial Continuous Prisoner's Dilemma. Journal of Theoretical Biology 231: 97-106
JAFFE K (2001) On the relative importance of haplo-diploidy, assortative mating and social synergy on the evolutionary emergence of social behavior. Acta Biotheoretica 49: 29-42.
JAFFE K (2002) An economic analysis of altruism: Who benefits from altruistic acts? Journal of Artificial Societies and Social Simulation 5: 3. https://www.jasss.org/5/3/3.html
JAFFE K (2004) Altruistic punishment or decentralized social investment? Acta Biotheoretica 52: 155-172.
JENSEN H J (1998) Self-Organizing Criticality. Emergent complex behavior in physical and biological systems. Cambridge Lecture Notes in Physics. University of Cambridge Press.
KURZBAN R and Houser D (2005) Experiments investigating cooperative types in humans: A complement to evolutionary theory and simulations. PNAS 102: 1803-1807
LEHMANN L and Keller L (2006) The evolution of cooperation and altruism — a general framework and a classification of models. J. Evol. Biol. 19: 1365-1725.
LIEBERMAN E, Hauert C and Nowak MA (2005) Evolutionary dynamics on graphs. Nature 433: 312-316.
LUMSDEN C.J. and Wilson E.O (1981) Genes, mind, and culture. Harvard University Press, Cambridge, MA
MAYNARD-SMITH J and Szathmary E (1997) The Major Transitions in Evolution. Oxford Univ. Press, Oxford.
NELSON R.R. and Winter S.G (1982) Evolutionary theory of economic change. Belknap Press. p. 454.
NOWAK M.A. and Sigmund K (1998) The dynamics of indirect reciprocity. J. Theor. Biol. 194: 561-574.
NOWAK M.A. and Sigmund, K (2004) Evolutionary dynamics of biological games. Science 303: 793-799.
NOWAK M.A. and Sigmund K (2005) Evolution of indirect reciprocity. Nature 437: 1291-1298.
OSBORN F and Jaffe K (1997) Cooperation vs. exploitation: interactions between Lycaenid (Lepidopera: Lycaenidae) larvae and ants. Journal of Research on the Lepidoptera. 34: 69-82.
OHTSUKI H, Hauert C, Lieberman E, Nowak M.A (2006) A simple rule for the evolution of cooperation on graphs and social networks. Nature 441:502-505
PANCHANATHAN K and Boyd R (2004) Indirect reciprocity can stabilize cooperation without the second-order free rider problem. Nature 432: 499-502.
PAGEL M. and Mace R (2004) The cultural wealth of nations. Nature 428: 275 - 278.
QUELLER D.C, Strassmann J.E. and Hughes C.R (1988) Genetic relatedness in colonies of tropical wasps with multiple queens. Science 242: 1155-1157.
Richerson P.J and Boyd R (2004) Not by Genes Alone: How Culture Transformed Human Evolution. Chicago Univ. Press 344 pp.
SANTOS F C, Rodrigues J.F and Pacheco J.M (2006) Graph topology plays a determinant role in the evolution of cooperation. Proc. Royal Soc. London B 273, 51-55.
SOULE T (2005) Resilient Individuals Improve Evolutionary Search. Artificial Life 12: 17-34
Return to Contents of this issue
© Copyright Journal of Artificial Societies and Social Simulation, [2007]